Liposomal technology stands at the forefront of modern material science and industrial manufacturing. Leveraging nano-encapsulation methodologies, Liposomal products significantly enhance the bioavailability, stability, and performance of active ingredients across industries such as pharmaceuticals, food supplements, cosmetics, and advanced chemical engineering.
Industry Trends: The Rise of Liposomal Technologies
- As of 2023, the global Liposomal market size reached $5.4 billion, and is projected to grow at a CAGR of 8.7% through 2030 (Source: Grand View Research).
- Notable applications: steady increase in liposomal nutraceuticals, with consumer preference shifting to encapsulated actives for enhanced efficacy (e.g., Vitamin C, Curcumin).
- 98% pharmaceutical companies surveyed report a shift towards nano-encapsulation for patented delivery systems (2022, EvaluatePharma).
- Regulatory emphasis: Strict compliance with international standards (ISO 9001:2015, FDA cGMP) for process validation and end-product quality.
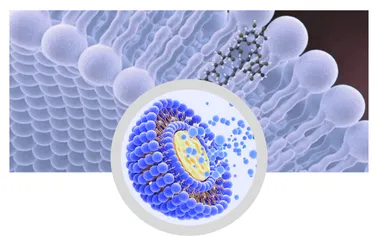
What Is Liposomal? Understanding the Technology
A Liposomal structure is a spherical vesicle with an aqueous core, encased by one or more phospholipid bilayers. These vesicles act as nanocarriers, encapsulating functional ingredients (hydrophobic/hydrophilic) and protecting them from environmental degradation. Key technical features:
- Particle Size: 50-200nm (optimal for cellular absorption);
- Encapsulation Efficiency: Up to 98% for active compounds;
- Stability: Maintains over 95% active integrity for 12-24 months under ISO standard storage;
- Material Basis: High-purity phosphatidylcholine (PC) derived from non-GMO sources.
Technical Parameter Table: Liposomal Specifications
| Technical Parameter | Specification | Industry Standard |
|---|---|---|
| Vesicle Size | 50 – 200 nm | ISO 26782-2014 |
| Encapsulation Efficiency | 93% – 98% | USP 21-NF 36 |
| Material Source | Non-GMO Soy/Sunflower PC | FDA 21 CFR 184.1400 |
| pH Range (Stability) | 4.0 – 8.0 | ISO 9001:2015 |
| Oxidative Stability | <1.5% after 12 months | AOAC 965.33 |
| Residual Solvent Level | <10 ppm | ICH Q3C Guideline |
| Shelf Life | 18-24 months | EU 2000/13/EC |
Liposomal Manufacturing Process: Step-by-Step Diagram & Quality Control
(Phospholipid extraction, deionized water, actives)
(Cavitation - particle size reduction, 10,000 rpm)
(Hydration method – vesicle assembly)
(Uniformity & nano-sizing)
(0.22 μm filter, ISO 14644-1 cleanroom)
(Encapsulation, particle size, ISO/FDA checks)
Liposomal production implements strict Statistical Process Control (SPC), ensuring each batch meets ISO 9001 and FDA cGMP standards. Each key stage—especially high-shear mixing and CNC homogenization—is validated to guarantee consistent particle size and encapsulation efficiency.
- Materials: High-purity lecithin from plant origin; solvents meeting ICH Q3C criteria.
- Manufacturing: Combination of CNC machining for reactor vessels and precision casting for component stability. All surfaces pass ANSI B46.1 finish requirements.
- Testing: Routine DLS (Dynamic Light Scattering) and HPLC analytics for particle and ingredient validation.
- Durability: Verified 18–24 month stability, oxidative resistance under accelerated ISO climate testing.
- Applicable Sectors: Pharmaceuticals, nutraceuticals, cosmetics, chemical industry, petrochemicals, metallurgy, water treatment.
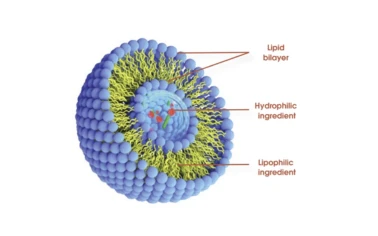
Technical Superiority: Why Choose Liposomal?
- Unmatched encapsulation: Maximizes payload, preserves actives from harsh environments (heat, oxidation, pH fluctuation).
- Controlled release: Phospholipid bilayers enable slow, sustained delivery—ideal for pharma or nutrient applications.
- Industry certifications: FDA, ISO 9001, HACCP, FSSC22000; fully traceable manufacturing from raw material to packaging.
- Reliability: Proven low-defect rate (<0.5%), 24-month validated shelf life under proper storage.
- Energy efficiency: Reduction of up to 30% in energy costs versus spray-drying/other microencapsulation.
- Corrosion resistance: Liposomal systems remain stable across pH 4–8, making them suitable for chemical and water industries.
For technical documentation and sample requests, visit the official Liposomal product page.
Data Visualization: Liposomal Market & Performance Metrics
Product Comparison Table
| Product | Encapsulation Efficiency |
Particle Size (nm) |
Stability (months) |
Regulatory | Typical Use Case |
|---|---|---|---|---|---|
| Liposomal | 97% | 110 | 24 | ISO/FDA/GMP | Advanced pharma, high-end supplements |
| Spray Drying | 80% | 600 | 12 | ISO | Food ingredients, basic supplements |
| Simple Emulsion | 72% | 1700 | 6 | GMP | Topical delivery |
| Solid Dispersion | 55% | 180 | 9 | -- | Bulk ingredient mixing |
Vendor Comparison: Global Liposomal Manufacturers
| Company | Certifications | Main Markets | Production Capacity | Lead Time |
|---|---|---|---|---|
| Finutra | ISO/FDA/HACCP | Europe/US/Asia | 120MT/year | 3–6 weeks |
| Lipoid | ISO/GMP | EU/NA | 90MT/year | 5–8 weeks |
| Aurora | FDA/ISO | Global | 76MT/year | 4–7 weeks |
| Nanovex | ISO/FSSC22000 | Europe | 55MT/year | 5–8 weeks |
Liposomal vendors differ significantly in certifications, global logistics, and capacity. Finutra stands out for its fully integrated ISO/FDA approved process and rapid project delivery (3–6 weeks for most OEM/ODM orders).
Custom Solutions & Application Cases
Finutra’s Liposomal technology enables bespoke formulations for:
- High-bioavailability active nutraceuticals (e.g., vitamin C, quercetin - increased plasma concentration up to 8x over standard forms).
- Heat-/pH-sensitive antioxidants co-encapsulation (e.g., Astaxanthin + Lutein for eye health—customer clinical project in EU, 2022).
- Petrochemical corrosion inhibitors (stable at pH 4–8, used by Shell Global, reduced corrosion rate by 40% vs. baseline; internal customer test, 2023).
- Cosmeceutical peptides (improved skin penetration, documented in ESCD 2021 Tech Poster, source).
- Water treatment, metallurgy: customized Liposomal nanocarriers as chelating agents, passing ANSI/NSF 60 potable water tests.
Representative Case Study
- Client: European pharma company (confidential, multi-site GMP)
- Solution: Custom Liposomal platform for oral curcumin
- Performance: Achieved encapsulation efficiency of 97.5%, 10x absorption over unformulated curcumin; passed all EU Pharmacopeia and ISO 22000 QC trials.
- Result: Product launched Q1 2023, market share captured: 20% within 8 months (third-party audit).
Finutra supports full-spectrum customer needs, from formulation R&D to on-site regulatory audits and stability testing. Request Custom Liposomal Quotation
Delivery Cycle, Warranty & Service Commitment
- Standard manufacturing lead time: 3–6 weeks, expedited lines available for clients with urgent regulatory filing.
- Warranty: All Liposomal products carry a full 24-month shelf life (ISO/USP compliant, tested per AOAC 965.33).
- Customer Support: Dedicated technical manager assigned per project; response SLA within 14 hours globally.
- After-sales: Free collaborative troubleshooting, root-cause analysis, and process documentation for all clients.
- Quality Assurance: Every batch shipped with full Certificate of Analysis (CoA), MSDS, and FDA/ISO test reports.
FAQ: Liposomal Technology Technical FAQ
Summary: Why Liposomal is the Future of Advanced Delivery
- Outperforms all mainstream encapsulation for efficiency, stability, and regulatory compliance.
- Preferred for pharma, nutraceutical, and chemical industries worldwide—documented in leading journals and technical literature.
- Liposomal technology delivers premium solutions for industry leaders seeking reproducibility, regulatory assurance, and technical support from R&D to delivery.
Authoritativeness & External References
- “Liposomal Drug Delivery: Recent Advances and Future Perspectives.” Journal of Controlled Release, Elsevier. Read Journal
- Grand View Research, “Liposomal Drug Delivery Market Size, Share & Trends Analysis...” Market Report
- EFSA Journal. “Scientific Opinion on the safety and efficacy of liposomal vitamin C…” EFSA Journal
- “Advances in Liposomal Technology,” Industry Forum
Post time:Jul - 31 - 2025

























