Understanding Advanced Liposomal Delivery Systems
In the rapidly evolving landscape of nutraceuticals and pharmaceuticals, the demand for enhanced bioavailability and targeted delivery of active compounds has never been greater. Traditional delivery methods often face challenges such as degradation by digestive enzymes, poor absorption, and rapid metabolism, significantly limiting the efficacy of many beneficial ingredients. This is where Liposomal technology emerges as a transformative solution. Liposomes are microscopic, spherical vesicles composed of one or more lipid bilayers, typically made from phospholipids, which are the same material that forms human cell membranes. This unique structural similarity allows them to encapsulate hydrophilic (water-soluble) and hydrophobic (fat-soluble) compounds, protecting them from harsh environments and facilitating their efficient transport across biological barriers. By mimicking natural cell structures, liposomes offer an unparalleled ability to deliver nutrients directly into cells, bypassing common gastrointestinal challenges and significantly increasing absorption rates. This innovative approach ensures that a higher concentration of the active ingredient reaches its intended target, maximizing its therapeutic potential and providing superior health benefits compared to conventional supplements. The versatility of liposomal formulations makes them ideal for a wide array of applications, from essential vitamins and minerals to complex botanical extracts and specialized pharmaceuticals, marking a significant leap forward in effective nutrient delivery.
The core advantage of Liposomal technology lies in its capacity to protect sensitive active ingredients from degradation while facilitating their absorption. For instance, many vitamins like Vitamin C are highly susceptible to oxidation and degradation in the stomach's acidic environment. Encapsulating such nutrients within liposomes shields them, ensuring their integrity until they reach the intestines where they can be absorbed more efficiently. Furthermore, the small size of liposomal vesicles, typically ranging from 50 nm to 200 nm, allows for their passive absorption across the intestinal lining, directly into the bloodstream and lymphatic system, bypassing the first-pass metabolism in the liver. This direct pathway means higher plasma concentrations of the active compound are achieved, leading to a more potent and faster-acting effect. The lipid bilayer can also be engineered with specific surface modifications to achieve targeted delivery to particular cells or tissues, reducing systemic side effects and improving therapeutic outcomes. This advanced targeting capability is particularly promising for pharmaceutical applications, offering precise drug delivery where it is most needed. The robust nature and customizable properties of liposomes position them at the forefront of modern delivery science, addressing critical bioavailability challenges across multiple health and wellness sectors.
The Advanced Manufacturing Process of Liposomal Formulations
The manufacturing of high-quality Liposomal products is a sophisticated process demanding precision engineering, stringent quality control, and specialized equipment to ensure optimal vesicle formation, stability, and encapsulation efficiency. The foundation of liposome production involves selecting high-purity phospholipids, often derived from soy or sunflower lecithin, which form the lipid bilayer. The active ingredient, whether water-soluble or fat-soluble, is then combined with these lipids in an aqueous solution. Several advanced manufacturing techniques are employed to create liposomes with desired characteristics, including controlled particle size and uniform distribution. One prominent method is thin-film hydration, where lipids are dissolved in an organic solvent, evaporated to form a thin film, and then rehydrated with an aqueous solution containing the active ingredient, followed by sonication or extrusion to reduce particle size. Another widely used technique is high-pressure homogenization, which involves passing the lipid-active ingredient mixture through a narrow orifice at very high pressures. This method effectively reduces vesicle size and creates more homogeneous liposomes, enhancing their stability and absorption properties.
Microfluidics represents an emerging and highly efficient method for Liposomal production, offering precise control over particle size and polydispersity index (PDI). In microfluidic systems, lipids and aqueous solutions are precisely mixed within microchannels, leading to self-assembly of uniform liposomes. This method allows for continuous production, high throughput, and reduced batch-to-batch variability, which is crucial for scalability and consistent product quality. Following the formation of liposomes, a series of rigorous detection and quality assurance standards are applied. Key parameters measured include particle size distribution using Dynamic Light Scattering (DLS), encapsulation efficiency (EE) to determine the percentage of active ingredient successfully encapsulated, and zeta potential to assess the surface charge and stability of the vesicles, which directly impacts their shelf-life and in-vivo behavior. Stability testing under various environmental conditions (temperature, humidity) is also critical to ensure product integrity over time. Compliance with international standards such as ISO (for quality management systems) and cGMP (current Good Manufacturing Practices) is paramount, ensuring that every batch meets the highest levels of safety, purity, and potency. The lifecycle of these advanced delivery systems is significantly extended through careful formulation and packaging, safeguarding their active contents.
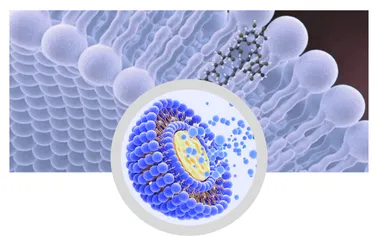
The meticulous selection of product materials, predominantly high-grade phosphatidylcholine, is crucial for forming stable and biocompatible liposomes. These phospholipids, often sourced from non-GMO sunflowers or soy, are vital for creating the bilayer structure that encapsulates and protects the active ingredients. The manufacturing process, irrespective of the chosen technique, also involves strict controls over environmental conditions, including temperature, pH, and sterility, to prevent contamination and maintain the integrity of the delicate liposomal structures. In essence, the entire process is a symphony of biochemistry and engineering, designed to yield a superior delivery vehicle. The applicable industries for such precisely engineered products span across pharmaceuticals, nutraceuticals, and cosmeceuticals, where the advantages of enhanced stability, improved bioavailability, and targeted delivery translate into significant benefits. For example, in nutraceuticals, Liposomal vitamins like Vitamin C or glutathione offer vastly superior absorption compared to traditional pills, preventing the degradation often seen in the digestive tract. This advanced protective capability, combined with efficient cellular uptake, ultimately leads to a more potent and effective product, benefiting consumers with enhanced health outcomes.
Key Technical Parameters and Advantages of Liposomal Formulations
The efficacy and quality of Liposomal formulations are defined by several critical technical parameters that differentiate them from conventional supplements. Understanding these metrics is vital for B2B decision-makers seeking to integrate superior delivery technologies into their product lines. Particle size is paramount; typically, liposomes range from 50 to 200 nanometers. Smaller, more uniform particles generally lead to higher absorption rates and improved stability due to their increased surface area to volume ratio and reduced tendency to aggregate. The polydispersity index (PDI) quantifies the uniformity of particle size distribution; a lower PDI (closer to 0.1) indicates a more homogeneous population of liposomes, which correlates with consistent performance and shelf-life. Encapsulation efficiency (EE) measures the percentage of the active ingredient successfully entrapped within the liposomes, directly impacting the effective dosage delivered. High EE ensures cost-effectiveness and maximal therapeutic benefit.
Another crucial parameter is Zeta Potential, which indicates the electrical charge on the surface of the liposomes and predicts their colloidal stability. A higher positive or negative zeta potential typically suggests greater electrostatic repulsion between vesicles, preventing aggregation and promoting a longer shelf-life. Stability, encompassing physical, chemical, and microbiological integrity over time, is evaluated through accelerated and real-time stability studies. These studies determine the product's shelf-life under various conditions, ensuring the active ingredient remains potent and the liposomes retain their structural integrity. When compared to conventional delivery systems, Liposomal formulations offer distinct advantages. Bioavailability is significantly enhanced, meaning a larger proportion of the active compound is absorbed and reaches systemic circulation, leading to higher therapeutic efficacy at lower doses. This is particularly beneficial for compounds with poor inherent bioavailability.
Moreover, liposomes provide excellent protection for sensitive active ingredients against degradation from stomach acids, enzymes, and oxidation, preserving their potency. They can facilitate targeted delivery, especially when surface-modified, allowing active compounds to reach specific cells or tissues, minimizing systemic exposure and potential side effects. The ability to encapsulate both hydrophilic and hydrophobic substances offers immense versatility, broadening the range of ingredients that can be effectively delivered. For instance, the use of Liposomal Vitamin C offers superior absorption compared to traditional ascorbic acid, with studies showing significantly higher plasma levels and cellular uptake. Similarly, for fat-soluble compounds like CoQ10 or Curcumin, liposomes dramatically improve solubility and absorption, overcoming their inherent insolubility challenges. These technical advantages translate directly into superior product performance and enhanced consumer benefits, making liposomal technology a preferred choice for high-value health products.
Key Technical Specifications of Advanced Liposomal Formulations
| Parameter | Typical Range for High-Quality Liposomal Formulations | Significance |
|---|---|---|
| Particle Size | 50 nm - 200 nm (optimal: 80-150 nm) | Smaller particles enhance cellular uptake, absorption, and stability. |
| Polydispersity Index (PDI) | < 0.2 (ideally < 0.15) | Indicates uniformity of particle size; lower PDI means higher consistency and stability. |
| Encapsulation Efficiency (EE) | Typically > 80% (varies by payload) | Percentage of active compound successfully encapsulated; higher EE indicates better formulation. |
| Zeta Potential | Typically +/- 20 mV to +/- 40 mV | Measures colloidal stability; higher magnitude (positive or negative) reduces aggregation. |
| Purity of Phospholipids | > 90% Phosphatidylcholine (PC) | Crucial for forming stable, biocompatible liposomal vesicles. |
| Shelf Stability (e.g., Vitamin C) | Retains > 90% potency over 12-24 months at specified conditions | Ensures product integrity and efficacy throughout its intended use period. |
Application Scenarios and Industry Trends for Liposomal Products
The versatility and superior performance of Liposomal technology have paved the way for its widespread adoption across numerous high-value application scenarios, transforming product efficacy in various industries. In the nutraceutical sector, liposomal delivery is revolutionizing supplements by significantly boosting the bioavailability of essential vitamins, minerals, antioxidants, and herbal extracts that traditionally suffer from poor absorption. For instance, liposomal Vitamin C, Curcumin, Glutathione, and CBD are now highly sought after due to their markedly improved cellular uptake and prolonged presence in the bloodstream, leading to enhanced health benefits. This is critical for consumers seeking maximum efficacy from their health investments, positioning liposomal supplements as premium-grade products. Beyond individual vitamins, complex formulations for immunity, cognitive health, and anti-aging are leveraging liposomes to deliver synergistic blends of ingredients more effectively. The demand for such advanced nutritional delivery systems is rapidly expanding, driven by increasing consumer awareness of bioavailability and a growing preference for natural, yet highly effective, health solutions.
In the pharmaceutical industry, Liposomal formulations are critical for delivering challenging drug compounds, including potent chemotherapeutics, antibiotics, and gene therapies. They help reduce systemic toxicity by enabling targeted delivery to specific disease sites, improving drug solubility, and extending drug circulation time in the body. For example, liposomal Doxorubicin (Doxil®) is a well-established example in cancer therapy, demonstrating reduced cardiotoxicity compared to free Doxorubicin. The cosmetic industry also benefits immensely from liposomes, using them to deliver active ingredients like hyaluronic acid, peptides, and retinoids deep into the skin layers, enhancing their anti-aging, moisturizing, and restorative effects. Emerging trends indicate a strong move towards personalized nutrition, where Liposomal technology can be tailored to individual needs, and the integration of smart release mechanisms for controlled delivery. The global market for liposomal drug delivery systems is projected to grow substantially, with a compound annual growth rate (CAGR) of over 10% in the coming years, driven by increasing R&D investments and expanding therapeutic applications.
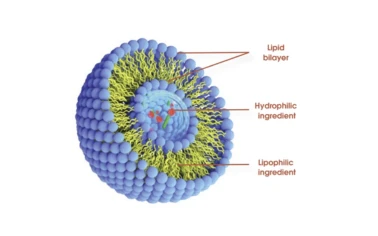
Further advancements are focusing on modifying liposomal surfaces with ligands for active targeting, enabling even more precise delivery to specific cells or tissues, minimizing off-target effects and maximizing therapeutic index. This precision makes Liposomal formulations particularly attractive for complex diseases, where localized delivery is paramount. Furthermore, the increasing interest in functional foods and beverages provides another burgeoning area for liposomes, where they can encapsulate sensitive bioactive compounds to improve their stability and absorption when incorporated into food matrices. The ability to encapsulate a broad spectrum of compounds, from highly sensitive enzymes to robust vitamins, positions liposomes as a foundational technology for future innovations across health, wellness, and medical sectors. Companies investing in liposomal technology are not just adopting a trend; they are embracing a scientifically proven method to deliver superior products that truly stand out in a competitive market, meeting the growing consumer demand for efficacy and quality.
Selecting a Liposomal Manufacturer and Custom Solution Benefits
Choosing the right manufacturing partner for Liposomal products is a strategic decision that directly impacts product quality, market competitiveness, and regulatory compliance. Expertise in liposome technology is paramount, encompassing deep scientific understanding of lipid chemistry, encapsulation techniques, and stability profiles. A reputable manufacturer should demonstrate a proven track record of successful liposomal formulation development and large-scale production, not just theoretical knowledge. Key considerations include the manufacturer's adherence to stringent quality control protocols, such as cGMP (current Good Manufacturing Practices) and ISO certifications, which are non-negotiable for ensuring product safety, purity, and consistency. Access to state-of-the-art analytical equipment for characterization (DLS, TEM, HPLC, GC-MS) and stability testing capabilities is also crucial, providing verifiable data on particle size, encapsulation efficiency, and shelf-life. Transparency in their process, from raw material sourcing to final product testing, builds trust and ensures that the end product meets the highest industry standards.
Furthermore, the ability of a manufacturer to offer bespoke Liposomal custom solutions is a significant differentiator. Generic liposomal formulations may not be optimized for specific active ingredients or desired release profiles, potentially compromising efficacy or stability. A custom solution involves tailored research and development, where experts work closely with clients to formulate liposomes specifically designed for their unique payload. This includes optimizing lipid composition, charge, particle size, and encapsulation method to achieve maximum encapsulation efficiency, stability, and bioavailability for the target compound. For instance, some ingredients may require specific pH conditions for optimal encapsulation, or different phospholipid ratios to enhance stability against oxidation. Customization extends to sensory attributes like taste and texture, and packaging considerations to ensure long-term product integrity. This tailored approach significantly enhances the product's market appeal and therapeutic performance.
Partnering with a manufacturer skilled in custom Liposomal solutions also offers strategic advantages such as intellectual property protection and a faster time to market for novel products. Their technical proficiency enables overcoming complex formulation challenges, transforming innovative concepts into tangible, high-performing products. The development process typically involves several stages: initial consultation and feasibility assessment, laboratory-scale formulation development and optimization, pilot-scale production, comprehensive analytical characterization, and finally, full-scale manufacturing. This iterative process, supported by robust data and scientific rigor, ensures that the final product not only meets but exceeds client expectations and regulatory requirements. Ultimately, an experienced manufacturer offering custom liposomal solutions acts as a true partner, contributing significantly to a brand's success by delivering cutting-edge products with unmatched bioavailability and efficacy.
Ensuring Trust: Quality Assurance, Support, and FAQ for Liposomal Products
For B2B partners, confidence in a Liposomal product provider stems from robust quality assurance, transparent operational practices, and reliable customer support. Authoritativeness is built upon adherence to the highest industry standards. This includes strict compliance with cGMP (current Good Manufacturing Practices) regulations, ensuring that products are consistently produced and controlled according to quality standards appropriate to their intended use. Certifications such as ISO 9001 for quality management systems further demonstrate a commitment to excellence across all processes, from research and development to manufacturing and distribution. Furthermore, rigorous third-party testing is crucial; independent laboratories verifying the potency, purity, and absence of contaminants (heavy metals, pesticides, microbial impurities) provide an unbiased guarantee of quality. Companies that openly share their Certificates of Analysis (CoAs) for each batch exemplify transparency and build significant trust with their partners. Consistent performance over years of service in the industry, backed by a strong portfolio of successful formulations, reinforces this authority.
Trustworthiness is also established through clear communication regarding lead times, delivery schedules, and comprehensive after-sales support. While delivery cycles can vary based on formulation complexity and order volume, a reputable provider will offer clear estimations and maintain open communication throughout the production and shipping phases, ensuring timely fulfillment. A robust quality guarantee or warranty commitment underscores a manufacturer's confidence in their Liposomal products. This typically includes assurances against manufacturing defects and a commitment to product stability and efficacy for a specified shelf-life. Exceptional customer support, including dedicated account managers, technical assistance for product integration, and responsive problem-solving, is vital for long-term partnerships. This ensures that any queries, technical challenges, or new product development ideas are met with expert guidance and prompt solutions, fostering a collaborative and reliable relationship.
To further enhance trustworthiness and provide immediate answers, a comprehensive FAQ section is invaluable:
Frequently Asked Questions (FAQ) about Liposomal Technology
-
Q: What makes Liposomal delivery superior to standard supplements?
A: Liposomal encapsulation protects active ingredients from degradation in the digestive system and significantly enhances their absorption into the bloodstream and cells, leading to much higher bioavailability and efficacy compared to traditional pills or powders. -
Q: Are Liposomal products safe?
A: Yes, liposomes are made from phospholipids, which are naturally occurring components of human cell membranes, making them highly biocompatible and safe for consumption. Reputable manufacturers adhere to strict safety and quality standards (e.g., cGMP). -
Q: How do you ensure the quality and stability of your Liposomal formulations?
A: We employ advanced manufacturing techniques, conduct rigorous in-process and final product testing (particle size, PDI, EE, zeta potential), and perform comprehensive stability studies. All processes adhere to cGMP and ISO standards, with third-party verification for purity and potency. -
Q: Can Liposomal technology be applied to any active ingredient?
A: While Liposomal technology is highly versatile and effective for a wide range of hydrophilic and hydrophobic compounds, successful encapsulation depends on the specific chemical properties of the active ingredient. Custom formulation development often optimizes this process for novel compounds.
Delivery Cycle & Warranty Commitment:
Our standard delivery cycle for bulk orders typically ranges from 4-6 weeks after final formulation approval, depending on raw material availability and order volume. Expedited options are available upon request. We provide a comprehensive quality warranty, ensuring our Liposomal products meet specified parameters and maintain stability for their declared shelf-life when stored under recommended conditions.
Our dedicated client support team is available to assist with technical queries, order tracking, and any specific requirements, ensuring a seamless partnership experience.
Concluding Insights: The Future of Nutrient Delivery
The journey through the intricate world of Liposomal technology reveals it as more than just a delivery mechanism; it is a fundamental advancement in maximizing the therapeutic and nutritional potential of various compounds. From its sophisticated manufacturing processes, ensuring precise particle size and high encapsulation efficiency, to its profound impact on bioavailability and targeted delivery, liposomes represent a paradigm shift in how active ingredients interact with the human body. The measurable improvements in absorption, stability, and efficacy offered by liposomal formulations are not merely incremental; they are transformative, providing a clear competitive edge in a market increasingly focused on tangible results. For B2B partners, integrating liposomal technology into their product portfolio means offering solutions that stand out for their superior performance, backed by rigorous scientific principles and advanced manufacturing capabilities. This commitment to innovation satisfies the growing consumer demand for highly effective and reliable health products.
As industry trends continue to emphasize personalized medicine, preventative health, and natural efficacy, the role of Liposomal technology will only expand. Its adaptability allows for the encapsulation of a diverse range of substances, from vitamins and minerals to complex botanicals and pharmaceuticals, making it a cornerstone for future product development. Partnering with a manufacturer proficient in both standardized and custom liposomal solutions ensures access to cutting-edge research, development expertise, and production capabilities that meet the highest regulatory and quality benchmarks. This strategic alliance guarantees that products are not only scientifically sound but also market-ready and highly competitive. The ongoing evolution of this technology promises even greater precision and efficacy, reinforcing its position as the gold standard for advanced nutrient and drug delivery, delivering profound benefits to both businesses and end-users.
References:
- Akbarzadeh, A., et al. "Liposome: classification, preparation, and application." Nanoscale Research Letters, 2013.
- Sercombe, L., et al. "Liposomes in drug delivery: A review." Pharmaceutics, 2015.
- Immordino, M. D., et al. "Stealth liposomes: Review of the basic science, rationale, and clinical applications, past and present." International Journal of Nanomedicine, 2006.
- Allen, T. M., & Cullis, P. R. "Liposomal drug delivery systems: From concept to clinical application." Advanced Drug Delivery Reviews, 2013.
- Sarkar, J., et al. "Liposomal vitamin C: A comprehensive review of its preparation, characterization, and therapeutic applications." Journal of Liposome Research, 2020.
Post time:Aug - 16 - 2025

























